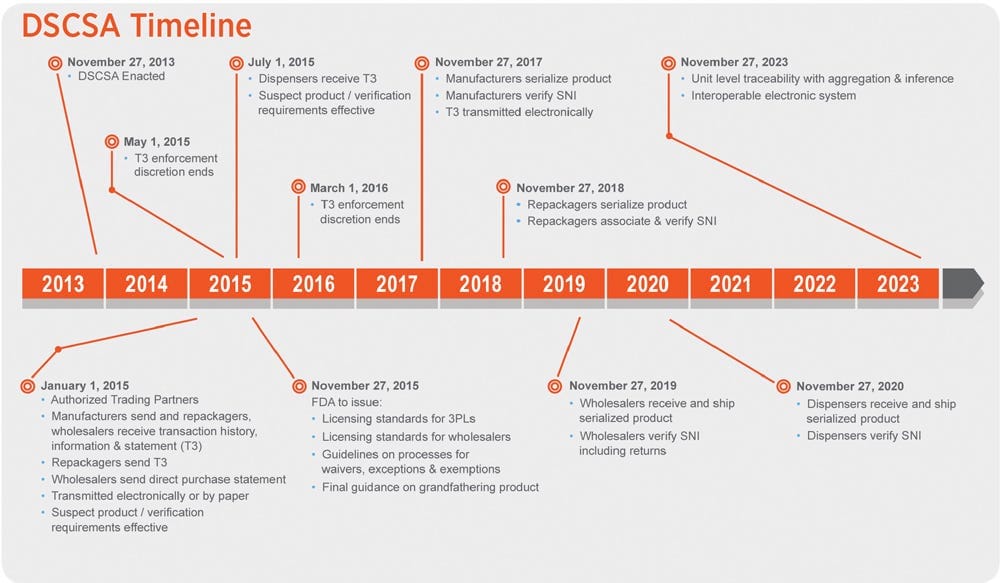
What Is DSCSA? 5 Requirements For Pharmacy Compliance


Clinical pharmacist Jennifer Griffin explains how Harps Food Stores successfully implements medical billing for clinical services, highlighting revenue cycle management, CPT codes, and workflow delegation.

Join SoftWriters at MHA Connect 2026 in Orlando, March 16–18! Visit booth #621 for FrameworkLTC® demos and catch CEO Scott Beatty and VP Danielle Greer discussing AI and the future of LTC pharmacy. Discover how our technology strengthens operations and improves resident outcomes.

Wondering what the excitement is about, when you have felt disruption, confusion, and frustration every day trying to get your team to implement the medication synchronization (med sync) workflow model? If this is your experience, then know that you are on the right path!

HERE WE GO AGAIN! ANOTHER ONE BITES THE DUST! PrimeRx Sold. QS/1 is end of life…. | Sponsored